Data-driven Process Systems Engineering Lab
Relevant Publications
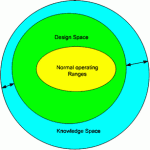
Comprehensive Quality by Design for Pharmaceutical Product Development and Manufacture 2018
The need for a more structured approach to process development has been recently identified in the pharmaceutical industry in order to consistently guarantee quality and value to processes and products. The concept of design space (DS) is a key aspect…
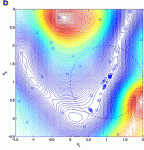
Journal of Pharmaceutical Information 2013
Simulation-based optimization is a research area that is currently attracting a lot of attention in many industrial applications, where expensive simulators are used to approximate, design, and optimize real systems. Pharmaceuticals are typical examples of high-cost products which involve expensive…
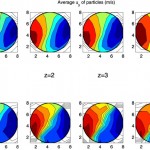
Chemical Engineering Science 2013
Reduced-order modeling (ROM) techniques are playing a very significant role in the recent literature bridging the gap between computationally expensive simulators and their application in optimization and control of distributed parameter systems. In modeling of solid-based processes, Discrete Element Method…
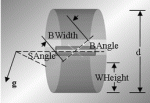
Journal of Pharmaceutical Innovation 2013
Purpose There has been increasing interest in the last few years especially within the pharmaceutical industry towards continuous powder blending. In this paper, the effects of different design and operating parameters are investigated, which include blade speed, shaft angle, weir…

Journal of Pharmaceutical Innovation 2013
In this work, a dynamic flowsheet model for the production of pharmaceutical tablets through a continuous wet granulation process is developed. The unit operation models which are integrated to compose the process line form a hybrid configuration which is comprised…
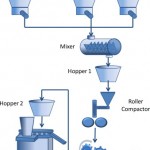
Computers & Chemical Engineering 2012
Manufacturing of powder-based products is a focus of increasing research in the recent years. The main reason is the lack of predictive process models connecting process parameters and material properties to product quality attributes. Moreover, the trend towards continuous manufacturing…

AAPS PharmSciTech 2012
A combination of analytical and statistical methods is used to improve a tablet coating process guided by quality by design (QbD) principles. A solid dosage form product was found to intermittently exhibit bad taste. A suspected cause was the variability…
Macromolecular Materials and Engineering 2012
The application of computationally inexpensive modeling methods for a predictive study of powder mixing is discussed. A multidimensional population balance model is formulated to track the evolution of the distribution of a mixture of particle populations with respect to position…
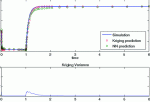
Industrial Engineering & Chemistry Research 2011
In many pharmaceutical process operations, first-principle models describing the behavior of the processed powders are difficult to derive or computationally very expensive and thus can be considered as “black-box” processes. Kriging is an efficient data-driven methodology that has been shown…

Journal of Pharmaceutical Innovation 2010
Introduction The identification and graphical representation of process design space are critical in locating not only feasible but also optimum operating variable ranges and design configurations. In this work, the mapping of the design space of pharmaceutical processes is achieved…
Relevant Presentations

2015 AIChE Annual Meeting in Exhibit Hall 1 (Salt Palace Convention Center)
November 08, 2015
As an engineering community we have been working towards developing detailed mathematical simulation models which accurately capture the behavior of complex systems. This trend is reinforced by our drive to couple multiscale information which ranges from the atomistic and molecular…

2012 AIChE Annual Conference in Pittsburgh
October 29, 2012
FDA harmonization guidelines [1] aim to promote building quality into design of pharmaceutical processes through mechanistic understanding of powder process behavior. Based on this paradigm, the industry is moving with a fast pace towards a model-based process and product design,…

2012 AIChE Annual Conference in Pittsburgh
October 31, 2012

International Forum of Process Analytical Technology (IFPAC) in Baltimore
January 24, 2012

2011 AIChE Annual Meeting in Minneapolis
October 17, 2011
The main purpose of this work is to develop the necessary tools required to integrate, simulate and design continuous manufacturing systems for pharmaceutical powder based products. The development of a detailed simulation will enable (a) the optimization of the integrated…

European Symposium on Computer Aided Process Engineering in Halkidiki, Greece
May 31, 2011

AIChE Annual Meeting in Salt Lake City
November 11, 2010
In emergent technologies it is very common that a functional form for the input-output relationships is unavailable and the process behavior is symbolically described using black-box models. This is the case for many pharmaceutical process operations, for which first-principles models…

AIChE Annual Meeting in Salt Lake City
November 08, 2010
The identification and graphical representation of process design space is very critical in locating not only feasible but also optimum operating variable ranges and design configurations. Design space of a process is the area of the parametric space within which…

2011 AIChE Annual Meeting in Minneapolis
October 20, 2011
The main purpose of this work is to develop an efficient strategy for simulation-based design and optimization for a pharmaceutical tablet manufacturing process. Simulation-based optimization is a research area that is currently attracting a lot of attention in many industrial…
