Data-driven Process Systems Engineering Lab
Design Space of pharmaceutical processes using data-driven based methods
Journal of Pharmaceutical Innovation 2010
Introduction
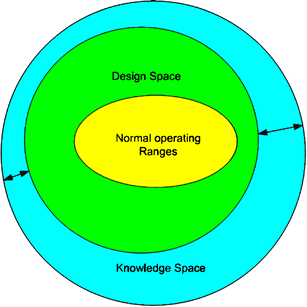
The identification and graphical representation of process design space are critical in locating not only feasible but also optimum operating variable ranges and design configurations. In this work, the mapping of the design space of pharmaceutical processes is achieved using the ideas of process operability and flexibility under uncertainty.
Methods
For this purpose, three approaches are proposed which are based on different data-driven modeling techniques: response surface methodology, high-dimensional model representation, and kriging methodology. Using these approaches, models that describe the behavior of the process at different design configurations are generated using solely experimental data. The models are utilized in mixed integer non-linear programming formulations, where the optimum designs are identified for different combinations of input parameters within the operating parameter and material property ranges.
Results
Based on this idea, by defining a desirable output range, the corresponding range of input variables that result to acceptable performance can be accurately calculated and graphically represented.
Conclusions
The main advantages of the methodologies used in this work are, firstly, that there is no restriction by the lack of first-principle models that describe the investigated process and, secondly, that the models developed are computationally inexpensive. This work can also be used for the comparative analysis of the use of different surrogate-based methodologies for the identification of pharmaceutical process Design Space.
