Data-driven Process Systems Engineering Lab
Methods and Tools for Design Space Indentification in Pharmaceutical Development
Comprehensive Quality by Design for Pharmaceutical Product Development and Manufacture 2018
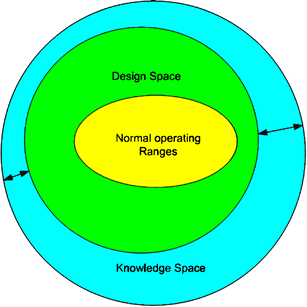
The need for a more structured approach to process development has been recently identified in the pharmaceutical industry in order to consistently guarantee quality and value to processes and products. The concept of design space (DS) is a key aspect of the pharmaceutical quality by design (QbD) initiative and was formally introduced in the International Conference on Harmonisation (ICH) Q8 guideline for pharmaceutical development. According to the formal definition, it is “the multidimensional combination and interaction of input variables (eg, material attributes) and process parameters that have been demonstrated to provide assurance of quality. Working within the design space is not considered as a change. Movement out of the design space is considered to be a change and would normally initiate a regulatory post approval change process.” The importance of the design space definition lies firstly in the “assurance of quality,” which is the key goal of QbD, and secondly in the broadening from an acceptable operating set point, to a collection of tolerable operating regions, which make up the DS. Thus, if a process DS is accurately identified, one can have the freedom to operate within this entire region without additional regulatory approval and simultaneously have higher confidence about the final product quality. For all the aforementioned reasons, the DS is a critical component of the recent effort of introducing QbD in pharmaceutical development and has attracted a lot of attention in the literature.
Link to Publication